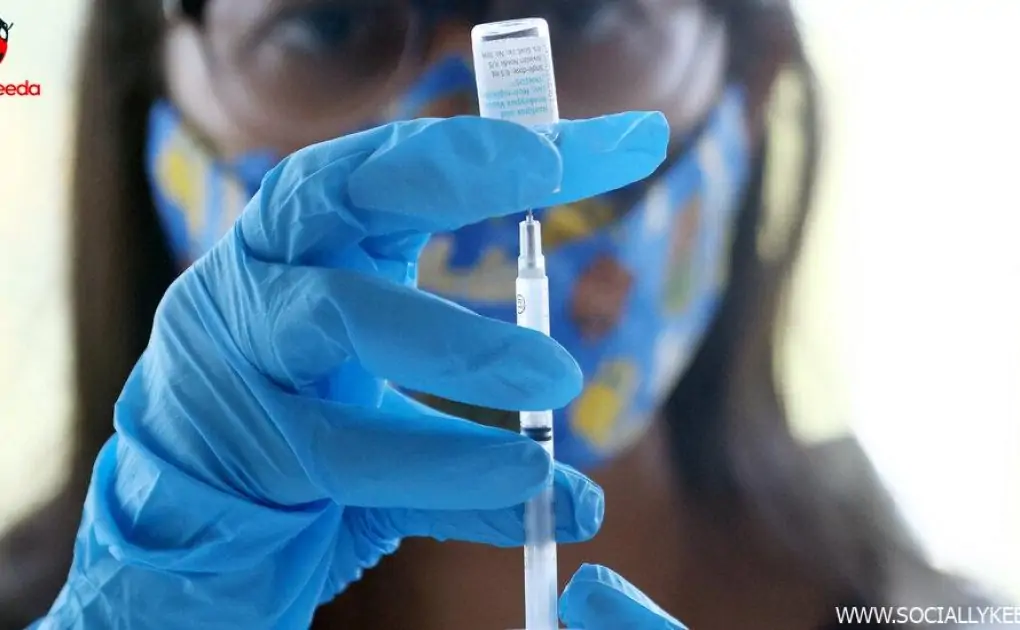
However the analysis could be very restricted. Scientists on the N.I.H. had deliberate to check the dose-sparing technique in a medical trial set to start in just a few weeks. It's unclear whether or not these plans can be shelved or sped up.
Details about how Jynneos performs in individuals with H.I.V., significantly in these with extreme immune issues, was already scant. In a single research carried out by Bavarian Nordic, antibody response to vaccination tended to be diminished: At 28 days after the primary shot, 67 p.c of these with H.I.V. produced antibodies, in contrast with 84 p.c of uninfected individuals.
Whereas Dr. Yonts mentioned the info from that trial was not conclusive, decreased antibody response is usually seen amongst immunocompromised individuals given different vaccines. Whereas evaluating Covid vaccines, for instance, researchers discovered that sufferers with H.I.V. have been more prone to have breakthrough infections.
“Individuals with severe or moderate immune suppression are recommended for additional doses of common vaccines,” mentioned Keri Althoff, an epidemiologist on the Johns Hopkins Bloomberg College of Public Health, who led the Covid vaccine research. “As immune suppression increases, the response to the vaccines does decrease.”
The C.D.C. and the New York Metropolis Division of Health say Jynneos is protected for individuals with H.I.V., however the businesses haven't addressed its effectiveness in that inhabitants.
In contrast, health officers in Britain say that for individuals who “are H.I.V. positive or have any other condition or treatment leading to a weakened immune system, the vaccine may not protect you as well.”